When an ‘alternative’ result is received that is more favourable the competency of the original laboratory is often called into question. This is both premature and unwise. The original result (while unwelcome) could be correct. There is no guarantee that the second result is correct either.
Comparison of the two results is counterproductive as they are likely to be two separate samples, both of which have ambiguous homogeneity in terms of the target analyte; if indeed, it was present at all in one or other of the samples.
When we consider that the analysis has taken place at a different location, using different equipment or different age and specification, at a different time of day with different environmental conditions and executed by different people it becomes easy to see how differences can occur.
Research into detection probabilities has quantified some of these uncertainties. Mortimer and Wallace1 looked at end product testing of milk powder contaminated with salmonella illustrating the variability of possible detection of a microbial hazard.
*Assuming detection test is 100% effective (most are <90%).
This is not to say that the laboratory has not made an error. Proficiency test schemes, which accredited laboratories participate in continue to show a proportion of 'poor' scores. A study by Elison et al2 identified that human error may be the core reason behind the poor proficiency scores, with sample preparation, human error, and equipment problems accounting for 41% of proficiency test variability.
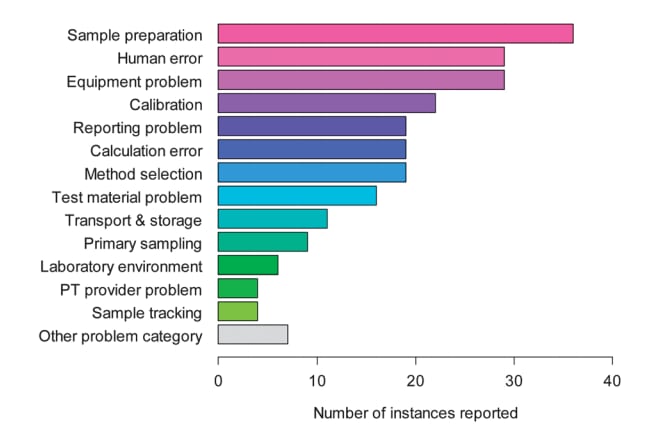
Fig 1. Causes of error in chemical analysis (Elison et al 2012)
How to handle an unexpected result
Rather than ‘retest’ upon receipt of an unfavourable result, an alternative could be to discuss the result with the laboratory provider in order to establish:
- Whether any difficulties were encountered during sample preparation
- What Internal quality control looked like for the sample
- When was the last external proficiency results for the target analyte were completed and how the laboratory performed?
- Who performed the analysis and when was there competence last assessed?
- Whether the laboratory has done any continuous improvement actions related to the target analyte?
- Whether there have been any recent instrumentation issues related to any part of the process?
- Does the result sit within the established range of quantification?
Another possibility is sending additional samples to an additional two alternative laboratories (to triangulate the result). Decisions can be made based on reasonable agreement between two of the three providers that is easier to justify than simply accepting a favourable result.
Ironically, exploring the validity of a result is seldom considered or explored if we get the result we want at the first time of asking.
- Mortimore, S.E. and Wallace, C.A.,1998, HACCP: a practical approach, Second Edition, Gaiththersburg, Aspen/Kluwer Publications.
- S. L. R. Ellison and W. A. Hardcastle, Accredit. Qual. Assur.,2012, 17, 453–464, DOI: 10.1007/s00769-012-0894-2.
Mark Hughes is technical services director at Food Forensics




