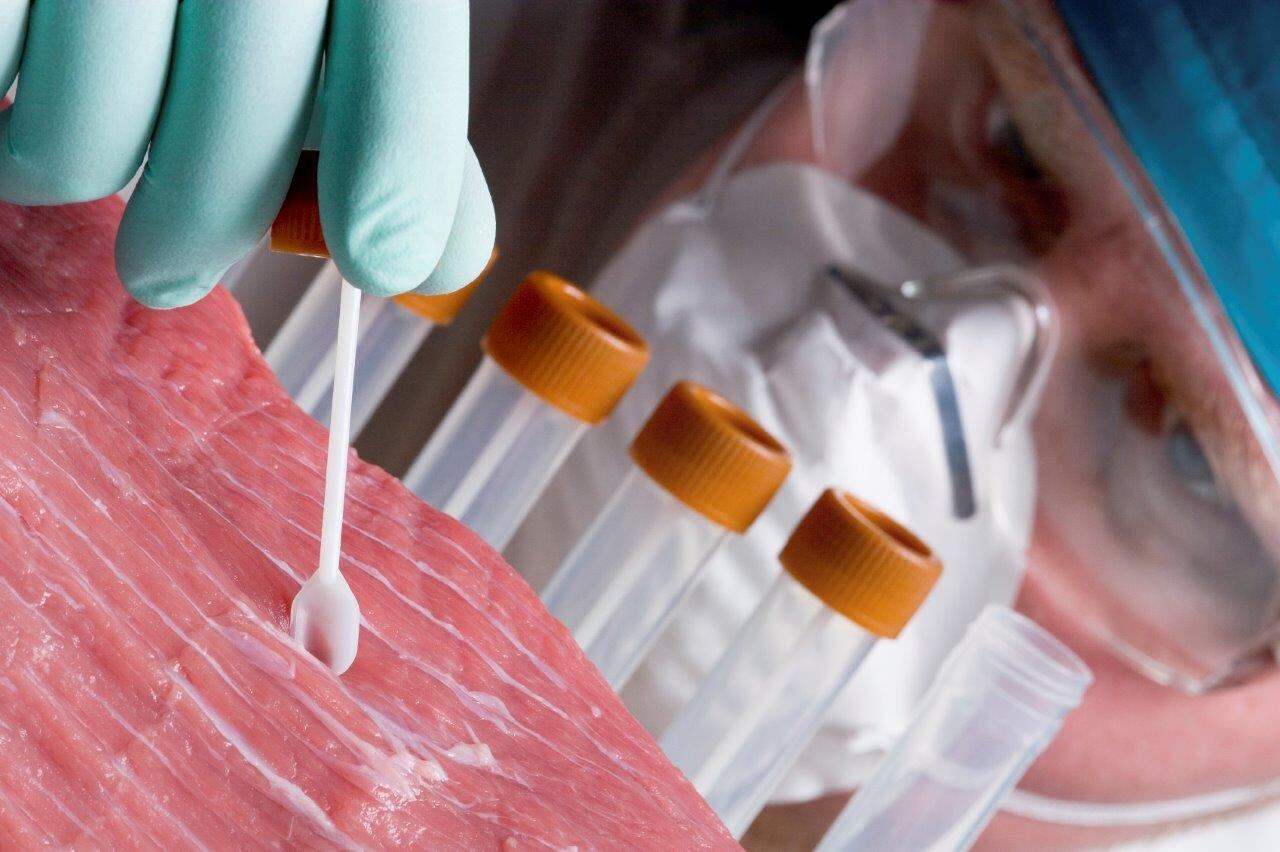
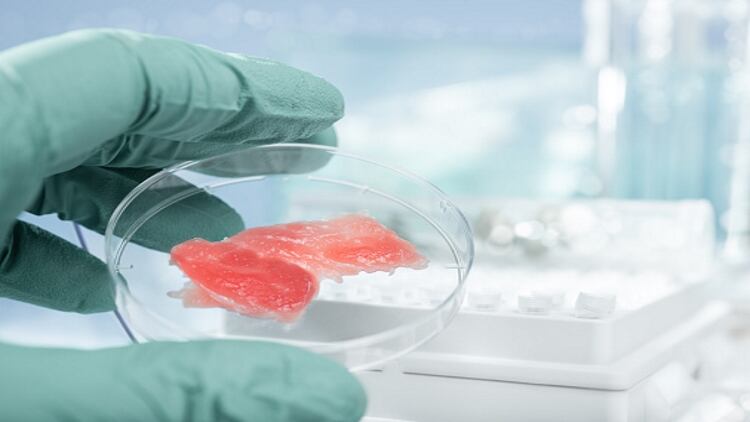
Long touted as a greener, more sustainable alternative to meat, challenges around lab-grown production include how to make large amounts of it and how to make it feel and taste more like the real thing.
To overcome these, researchers at the Harvard John A Paulson School of Engineering and Applied Sciences (SEAS) developed a technique known as immersion Rotary Jet-Spinning, which uses centrifugal force to spin long nanofibres of specific shapes and sizes.
The research is published in Nature Science of Food.
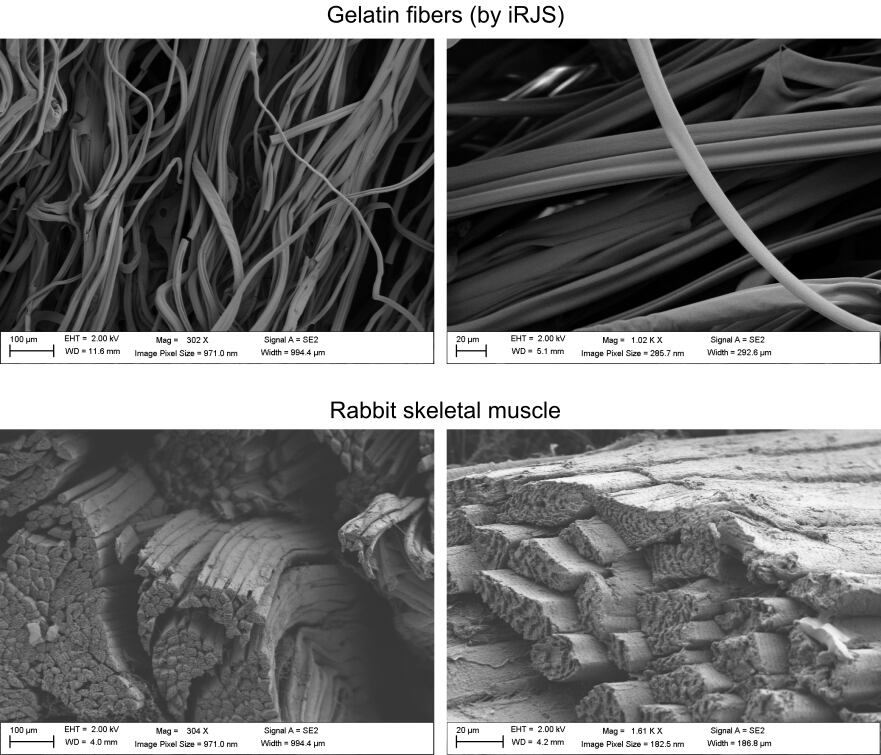
In this instance, the team spun food-safe gelatine fibres to form the base for growing cells. The fibres mimic natural muscle tissue’s extracellular matrix – the glue that holds the tissue together and contributes to its texture.
Researchers then seeded the fibres with rabbit and cow muscle cells, which anchored to the gelatine and grew in long, thin structures, similar to real meat.
Mechanical testing was used to compare the texture of their lab-grown meat to real rabbit, bacon, beef tenderloin, prosciutto and other meat products.
How animal meat is formed
Animal meat consists mostly of skeletal muscle (and fat tissue), which grow in long, thin fibres – as can be seen in the grain of a steak or when shredding pork or chicken. Reproducing these fibres is one of the biggest challenges in bioengineering meat.
“Muscle cells are adherent cell types, meaning they need something to hold onto as they grow,” said Luke MacQueen, first author of the study and research associate at SEAS and the Wyss Institute for Bioinspired Engineering.
“To grow muscle tissues that resembled meat, we needed to find a ‘scaffold’ material that was edible and allowed muscle cells to attach and grow in 3D. It was important to find an efficient way to produce large amounts of these scaffolds to justify their potential use in food production.”
When the team analysed the microstructure and texture, it found that, although the cultured and natural products had comparable texture, natural meat contained more muscle fibres, meaning they were more mature.
“Muscle and fat cell maturation in vitro are still a really big challenge that will take a combination of advanced stem cell sources, serum-free culture media formulations, edible scaffolds such as ours, as well as advances in bioreactor culture methods to overcome,” said MacQueen.
Why it might be possible to design meats
Still, this research showed that full lab-grown meat was possible, he added.
“Our methods are always improving and we have clear objectives because our design rules are informed by natural meats. Eventually, we think it may be possible to design meats with defined textures, tastes, and nutritional profiles – a bit like brewing,” said MacQueen.
Kit Parker, the Tarr Family professor of bioengineering and applied physics at SEAS and senior author of the study, said that moving forward, the goals were nutritional content, taste, texture and affordable pricing.
“The long-range goal is reducing the environmental footprint of food,” he said.
Kate Krueger, research director at New Harvest, a cellular agriculture research institution, who was not involved in the research, said the technique showed “great promise”.
“The development of cultured meat involves a number of technical challenges, including the formulation of a scaffold material that can successfully support cells and the development of cell lines that are amenable to cultivation for consumption at scale,” she said
“The authors of this publication have developed scaffold materials that show great promise in these areas.”
Harvard’s Office of Technology Development has protected the intellectual property relating to the project and is exploring commercial opportunities.
The research was supported by SEAS, the Wyss Institute for Biologically Inspired Engineering, Harvard Materials Research Science and Engineering Center and the TomKat Foundation.